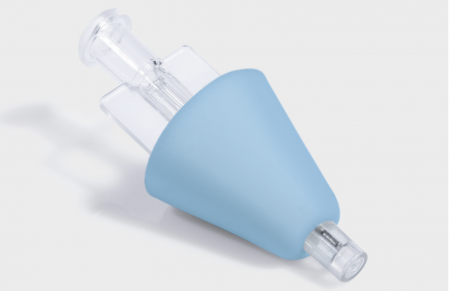
How Can Emergency Departments Reduce Needle-Stick Injuries While Ensuring Rapid Drug Delivery?
The MIRAD intranasal atomisation device eliminates needle-stick risks entirely while delivering medications through rapid mucosal absorption. Our ISO 13485 and FDA-certified system provides emergency departments with a safer, faster alternative for critical drug administration, reducing occupational hazards and improving patient outcomes. Contact us to discover how MIRAD can transform your emergency medication protocols.
With 28 years of medical device manufacturing expertise, our MIRAD system combines advanced atomisation technology with user-friendly design to ensure consistent, reliable drug delivery in time-critical situations. The device's non-evasive approach makes it particularly suitable for pediatric, geriatric, and needle-phobic patients, while its single-size specification simplifies inventory management for healthcare facilities. Backed by comprehensive quality control following IQC, IPQC, FQC protocols and AQL 1.0 S4 standards, each MIRAD unit undergoes rigorous testing and in-house EO sterilization to guarantee optimal performance and patient safety in anesthesiology, emergency medicine, and critical care applications.